Self Emulsifying Drug Delivery system
Review on Self Emulsifying Drug Delivery system -The oral route is most preferred route for drug delivery in human beings. Probably at least 90% of all drugs are administered through oral route. Oral delivery of 50% of the drug compounds is facing problems because of the low hydrophilicity(high lipophilicity) and less permeability of the drug . The solubility issues of orally administered drugs are a major challenge of pharmaceutical industry .
Drugs with low aqueous solubility have poor dissolution and low bioavailability. This can be increased by different methods like solid dispersions , salt formation ,complex formation . micronization , nanosuspension , modification of the crystal habit like polymorphs, amorphous form and cocrystallization
Self-Emulsifying Drug Delivery System (SEDDS) is one such methods used for improving the solubility of lipophilic drugs.SEDDS are isotropic mixtures of solvents and co-solvents/surfactants , surfactants ,oils, , can be used for the design of formulations in order to improve the oral absorption of drugs with low hydrophilicity (highly lipophilicity) SEDDS formulations are characterized by in vitro lipid droplet sizes of 200 nm–5 mm and the dispersion is turbid in appearance. It can be orally administered in soft or hard gelatin capsules. for ease of administration.
However, certain problems such as leaking, leaching of components from the capsule shell, and interaction of SES with capsule shell components are quite common. Sorption and permeation by the capsule shells has also been observed for such liquid-filled capsules. To sort out above listed problems, now a days SE system is being formulated and dispense in the form of solid and semi solid dosage forms.
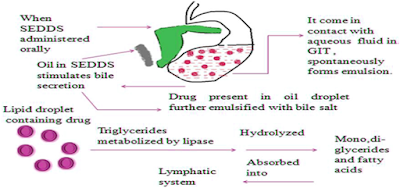
When orally administered, these systems form fine (micro or nano) emulsions in gastrointestinal tract (GIT) with mild agitation provided by gastric mobility (Charman et al., 1992. Shah et al., 1994, Pouton, C.W., 1997). So called as in situ emulsification or self-emulsification which further leads to solubilization of drug that can subsequently be absorbed by lymphatic pathways, bypassing the hepatic first-pass effect.
‘SEDDS’ is a broad term, typically producing emulsions with a droplet size ranging from a few nanometers to several-microns.
Self-Nano Emulsifying Drug Delivery System (SNEDDS) :
These are nano-emulsions formed by SEDDS. They are heterogeneous dispersions of two immiscible liquids (water-in-oil [W/O] or oil-in-water [O/W]) with a mean droplet size in nanometers (ranging from 20–200 nm).(2)Self-Micro Emulsifying Drug Delivery System (SMEDDS) :
These are micro-emulsions formed by the SEDDS. They exhibit thermodynamic stablility and forms transparent emulsion. Particle size of droplet is the main difference between micro-emulsions and common emulsions . The size of the droplets of common emulsion is between 0.2 to 10 μm, and size of droplets of micro-emulsion formed by the SMEDDS generally ranges between 2 and 100 nm. As the particle size is small, the total surface area for absorption and dispersion is significantly larger than that of solid dosage form and it can easily penetrate the GIT and gets absorbed. Hence the bioavailability of drugs is therefore improved .(3)Properties of Self Emulsifying Drug Delivery system (SEDDS) :
1. They rapidly self-emulsify in gastro-intestinal fluids and form a fine o/w emulsion. under the influence of mild agitation provided by peristaltic and other movements of gastro intestinal tract(GIT),
2. They can effectively incorporate either hydrophilic or hydrophobic drug within the mixture of oil-surfactant
3. They can be used for solid as well as liquid dosage forms.
4. They require lesser dose of drug when compared to conventional dosage forms. (4)
Advantages of Self emulsifying drug delivery system over conventional drug delivery systems :
1. Fine oil droplets of SMEDDS would pass rapidly throughout the stomach facilitating wide distribution of the drug throughout Gastro intestinal tract, thereby the irritation is minimized which is frequently encountered during extended contact between bulk drug substance and the wall of gut.
2. Emulsions are metastable and sensitive dispersed forms while SMEDDS are physically stable formulations.
3. When compared with oily solutions, they provide a large interfacial area for partitioning of the drug between oil and water.
4. Potential advantages of these systems include increased oral bioavailability, more accurate temporal profiles of drug absorption, selective drug targeting toward a specific absorption window in the GI tract, and drug protection from the averse environment in the gut. Thus, for lipophilic drug compounds that exhibit limited absorption, these systems may offer enhancement in the extent and rate of absorption.
5. They require very simple and economical manufacturing facilities like simple mixer with agitator and volumetric liquid filling equipment for large-scale manufacturing Ease of manufacture and scale- up is one of the most important advantages that make SMEDDS unique when compared to other drug delivery systems like solid dispersions, liposome, nanoparticles, etc (5)(6)
Disadvantages of Self Emulsifying Drug Delivery Systems :
1. Lack of good predicative in vitro models for assessment of the formulations is one of the obstacles for the development of SMEDDS and other lipid-based formulations.
2. These formulations potentially are dependent on digestion prior to release of the drug and hence traditional dissolution methods do not work.
3.Chemical instabilities of drugs and high surfactant concentrations in formulations (approximately 30-60%) which irritate GIT is another drawback of these systems.
4. Volatile co-solvents in the conventional SMEDDS formulations are known to migrate into the shells of soft or hard gelatin capsules, which causes precipitation of the lipophilic drugs.
5. Validation is more challenging as formulations contain several components.
6. Low drug incompatibility.
7. High production costs.
8. Drug leakage may allow less drug loading. (5)(6)
Composition of Self Emulsifying Drug Delivery System:
1. Active Pharmaceutical Ingredient (API):
As, SEDDS are used to increase the solubility of drugs with poor water solubility, BCS class II drugs e.g. itraconazole, nifedipine, vitamin E, simvastatin, danazol, ketoconazole, mefanimic acid, naproxen, carbamazepine are preferred (7)(8)
2. Excipients used in Self Emulsifying Drug Delivery system :
By considering issues of toxicity and pharmaceutical acceptability the selection of excipients is really a tough challenge. So there is a great restriction regarding the use of excipients .The self-emulsification process is specific to the concentration and nature of the surfactant/co-surfactant ratio , oil/surfactant ratio and the temperature at which self-emulsification occurs. Hence these factors must be taken under consideration during excipients selection in Self Emulsifying Drug Delivery system .
a. Oils : Required dose of the lipophilic drug can be solubulized by oils and facilitate self-emulsification and also they can enhance the amount of lipophilic drug transported through the intestinal lymphatic system,absorption of drug is increased from the GI tract depending on the molecular nature of the triglyceride . Both long chain triglyceride(LCT) and medium chain triglyceride (MCT) oils with different degrees of saturation are used for the design of self-emulsifying formulations. Novel semisynthetic MCT, which are amphiphilic compounds with surfactant properties, are progressively and effectively replacing the regular MCT oils in the SMEDDS. MCTs are highly soluble and have a greater mobility in the lipid/water interfaces when compared to LCT associated with a more rapid hydrolysis of MCT. (9)
When using LCT, a higher concentration of non-ionic solubilizers and emulsifying agents like cremophor RH40 is required to form microemulsions compared with MCT. Edible oils are not selected due to they are unable to dissolve larger amounts of lipophilic drug. Modified or hydrolyzed vegetable oils are widely used as these excipients form good emulsification systems with a large number of surfactants that are approved for oral administration and exhibit better drug solubility properties . They offer formulative and physiological advantages and their degradation products are similar to the natural end products formed in intestinal digestion.
b. Surfactants (10,11): Several compounds exhibiting surfactant properties can be used for the design of self-emulsifying systems, but the surfactants used must be orally acceptable. The most widely used ones are non-ionic in nature. The most widely recommended ones are the non‑ionic surfactants with a relatively high HLB.Safety is a major factor in choosing a right surfactant
The four main groups of surfactants are defined as follows:‑
• Anionic surfactants
• Cationic surfactant
• Ampholytic surfactants
• Non‑ionic surfactants.
(a) Anionic surfactants:‑In these surfactants the hydrophilic group carries a negative charge such as carboxyl (RCOO‑), sulfonate (RSO3 ‑) or sulfate (ROSO3 ‑). Examples are potassium laurate and sodium lauryl sulfate
(b) Cationic surfactants:‑ In these surfactants the hydrophilic group carries a positive charge. Example is quaternary ammonium halide
(c) Ampholytic surfactants(Also called zwitterionic surfactants):‑ These surfactants Contain both a negative and a positive charge. Example is sulfobetaines
(d) Non‑ionic surfactants:‑ In these surfactants the hydrophilic group carries no charge but derives its water solubility from highly polar groups like hydroxyl or polyoxyethylene. Examples are poly ‑sorbates (Tweens) and sorbitan esters (Spans)
c. Co-surfactants:
In most of the cases, single chain surfactants alone are unable to reduce the oil water interfacial tension to sufficient level to enable a microemulsion to formation. Thus a co- surfactant which is usually a medium chain fatty alcohol, acid or amine taken along with the surfactant to lower the interfacial tension to a very small or even transient negative value. At this value fine droplets get formed due to the interface expansion and more of surfactant/co-surfactant get adsorbed on the surface until the bulk condition is depleted enough to make the interfacial tension positive again. This process called the spontaneous emulsification forms the microemulsion.
Cosurfactants have an effect of further reducing the interfacial tension by the ‘dilution effect‘ whilst increasing the fluidity of the interface by decreasing the rigidity constant K, thereby increasing the entropy of the system.They also increase the mobility of the hydrocarbon tail and allow greater penetration of the oil into this region.
Any alcohol also influences the solubility properties of the aqueous and oily phases due to its portioning between these phases.
Many researchers used medium chain alcohols like pentanol and hexanol, but they are not of pharmaceutical grade and are not used in drug delivery due as they cause high irritation and also their volatile nature can destabilize the system. Hence non-ionic surfactant that are less irritant (polyoxyethylene alcohol esters) was used by many researchers for use as co-surfactant. Polyethylene glycol derivative of distearoyl phosphatidyl ethanolamine, ethanol, oleic esters of polyglycerol, fatty acid esters of propylene glycol, ethyldiglycol and polyethylene glycol were also evaluated as co-surfactants in micro and nanoemulsions drug delivery system (Dalmora et al., 2001; Kawakami et al., 2002). They help dissolve larger quantities of either the hydrophilic surfactant or the drug in the lipid base and can act as co-surfactant in the self-emulsifying drug delivery systems[SEDDS]
Some researchers also used alcohol- free self-emulsifying micro emulsions .Such systems may exhibit some advantages over the other formulations when incorporated in capsule dosage forms, As alcohol and other volatile co-solvents in the conventional self-emulsifying formulations are migrate into the shells of hard gelatin or soft sealed gelatin capsules which leads to precipitation of the lipophilic drug.
On the other hand, the lipophilic drug dissolution ability of the alcohol free formulation is limited. (12-17)
d. Viscosity Enhancers: The viscosity of the emulsions can be enhanced by the use of additional material such as acetyl alcohol, tragacanth, beeswax and stearic acids etc.
e. Polymers: Polymer matrix (inert) present in 5 to 40% w/w is non ionizable at physiological pH are able to form matrix. Examples are hydroxyl propyl methyl cellulose, ethyl cellulose, etc.
f. Antioxidant Agents: Lipophilic antioxidants (E.g. α tocopherol, propyl gallate, ascorbic palmitate) stabilize the oily content of SEDDS formulations.
Factors affecting SMEDDS :
1. Nature and dose of the drug:
Drugs which are taken in very high dose are not suitable for SMEDDS unless they exhibit good solubility in lipophilic phase(preferable) or in atleast one of the components of SMEDDS,. The drugs with lesser solubility in water and lipids typically with log p values of approximately 2 are difficult to deliver through SMEDDS. The solubility of drug in oil phase greatly influences ability of SMEDDS to maintain the drug in solubilised form..
2. Concentration of Surfactant or Co-surfactant:
There is a risk of precipitation If surfactant or co-surfactant is contributing to the greater extent in drug solubilization.as dilution of SMEDDS results in lowering of solvent capacity of the surfactant or co-surfactant.
3. Polarity of the Lipophilic phase:
Drug release from microemulsion is governed by the polarity of the lipid phase .The droplet polarity is governed by the HLB, the chain length and degree of unsaturation of the fatty acid, the molecular weight of drug that is micronized. (18)
The Emulsification Process:
1. Mechanism of Self-emulsification:
Mechanism of Self Emulsifying Drug Delivery system SEDDS
Different approaches have been reported . But no single theory explains all aspects of micro emulsion formation. As per the investigations of Schulman et al. the spontaneous formation of micro emulsion droplets was due to the formation of a complex film at the interface of oil‐water by the surfactant and co‐surfactant. Thermodynamic theory of formation of micro emulsion explains that emulsification occurs, when the entropy change that favour dispersion is greater than the energy required to increase the surface area of the dispersion and the free energy (ΔG) is negative. The free energy in the micro emulsion formation is a direct function of the energy required to create a new surface between the two phases and can be described by the equation
ΔG = Σ Nᴫ r2 σ
Where,
ΔG is the free energy associated with the process (ignoring the free energy of the mixing).
N is the number of droplets of radius r
σ are presents the interfacial energy.
With time, the two phases of the emulsion tend to separate as a result the interfacial area is reduced and eventually , the free energy of the system decreases. Therefore, the emulsion resulting from aqueous dilution are stabilized by conventional emulsifiers. Emusifying agents form a mono layer around the emulsion droplets, and hence the interfacial energy is reduced, and also providing a barrier to prevent coalescence.(19-22)
2. Construction of Ternary Phase Diagrams:
Pseudoternary diagrams are often constructed for the development of Self Emulsifying Drug Delivery system SEDDS Comparison of different surfactants and their synergy with co-solvent is enabled using equilibrium phase diagram. The boundaries of one phase region can be assessed visually and a ternary phase diagram represents phase behaviour of a three-component system.
A phase diagram helps in determining the optimum concentrations of different excipients required to obtain homogenous pre-concentrates, self-emulsifying ability and drug loading. Each corner of phase diagram represents 100% of particular components and when more than three components are used, closely related one are grouped as one component and treated as such in the diagram(23)
Evaluation of Self Emulsifying Drug Delivery system (SEDDS):
A number of tests are carried out for characterization and evaluation of Self Emulsifying Drug Delivery system (SEDDS). (24) (25)
1. Drug Content:
SEDDS are pre-weighed and drug is extracted from SEDDS by dissolving in suitable solvent. Drug content in the solvent extract is analyzed by suitable analytical method .
2. Dispersibility Test:
The dispersibility test of SEDDS is carried out to find its capability to disperse into emulsion and categorize the size of resulting globules. It is carried by using a standard USP dissolution apparatus (Paddle Type).
One ml of each formulation is added to 500 ml of water at 37 + 0.5ºC and the paddle is rotated at 50 rpm. On titration with water the SEDDS formulation forms a mixture or gel which is of different type depending upon which the in vitro performance of formulation can be assessed using the following grading system
Grade A: Rapidly forming (within 1 min) nanoemulsion, having a clear or bluish in appearance.
Grade B: Rapidly forming, slightly less clear emulsion, having a bluish white in appearance.
Grade C: Milky fine emulsion that formed within 2 min.
Grade D: Dull, greyish white emulsion having slightly oily appearance that is slow to emulsify which takes usually longer than 2 min.
Grade E: Formulation, exhibiting either poor or minimal emulsification with large oil globules present on the surface.
Grade A and Grade B formulation will remain as nanoemulsion when dispersed in gastro intestinal tract . Formulation in Grade C could be recommended for SEDDS formulation. The stability of the formulation decreases from micro emulsion to emulgel given below
TYPE OF FORMULATION DEPENDING UPON VISUAL OBSERVATION
Type of formulation Mixture/Gel
Micro emulsion Transparent mixture
Micro emulsion gel Transparent Gel
Emulsion cloudy mixture
Emulgel Milky Gel
3. Rheological properties determination:
The SEDDS system can also be incorporated in soft gelatin capsules, where, it should have appreciable flow properties for processing. The rheological properties (viscosity, flow, thixotropy, static yield, creep value) of formulation (diluted to 5 % v/v water) are determined by rotational viscometers, digital instruments coupled with either cup and bob or coaxial measuring device.
A type of rotational viscometer has also been used for finding of viscosity of fresh and other SEDDS formulations which has been stored for longer durations .
Determination of Viscosity of liquid SEDDS also indicates whether the system is o/w or w/o. Viscosity of formulation is inversely proportional to dilution. (26)
Low viscosity systems are o/w and high viscosity systems are usually w/o in nature. .
Thermodynamic stability studies:
The physical stability of a formulation is very essential for its performance as it can be adversely affected by precipitation of the drug in the excipient matrix. Poor physical stability of formulation leads to phase separation of excipients which affects bioavailability and therapeutic efficacy. Also the incompatibilities between formulation and gelatin capsule shell(if formulation filled in capsule) may cause softness ,brittleness, and disintegration is delayed or drug is incomplete drug release. The cycles carried out for these studies are as follows
a. Heating cooling cycle:
Six cycles of cooling and heating are carried between refrigerator temperature of 4°C and elevated temperature of 45°C with exposure at each temperature for not less than 48 hours . The stable formulations are then subjected to centrifugation test.
b. Centrifugation:
Formulations which pass the heating-cooling cycle are subjected to centrifugation at 3500 rpm for 30 mins. The formulations which do not show any phase separation are taken for freeze thaw stress test.
c. Freeze thaw stress cycle:
Three freeze thaw cycles between -21° C & 25° C . The formulations which pass this test show good stability with no phase separation, cracking or creaming. The formulations that pass this test are then further subjected to dispersibility test for assessment of self-emulsification efficiency.
5. Robustness to Dilution:
On dilution with different dissolution media emulsions should not show any phase separations or precipitation of drug even after 12 hrs of storage, such formulations are considered as robust to dilution .
6. Turbid Metric Evaluation:
Turbidity is a parameter for determining droplet size and self-emulsification time 19 .Fixed amount of SEDDS is added to fixed quantity of suitable medium (0.1 N Phosphate Buffer or HCL) under continuous stirring at a rate of 50 rpm using magnetic stirrer at optimum temperature and the turbidity is measured using a turbidimeter. Since the time required for complete emulsification is too short, it is not possible to monitor the rate of change of turbidity i.e. rate of emulsification. Turbidimetric evaluation is carried out to monitor the growth of droplet after the process of emulsification.
7. Droplet size analysis & Particle size measurements:
Droplet size is an important factor in the self‑emulsification process because it determines the rate of drug release and extent of drug release as well as absorption.Dynamic Light scattering (DLS) ,Photon correlation spectroscopy (PCS) , Laser Diffraction Techniques and coulter nanosizer are used to determine droplet size of emulsion. Equipments like Mastersizer, Particle Size Analyzer, Zetasizer which are able to measure sizes between 10 and 5000 nm are used for for measurement of particle size.
8.Zeta potential
This is used to find the charge on the droplets. The charge on the oil droplets is due to inconventional SMEDDS.The charge is negative due to the presence of free fatty acids. Incorporation of a cationic lipid (oleylamine) at a concentration range of 1‑3% will yield cationic SMEDDS. Zeta potential helps to predict the stability and flocculation effect in emulsion systems. A high zeta potential maintains a stable system . If the zeta potential falls below a certain level, colloid will aggregate due to attractive forces.
9. Self-Emulsification Time:
The self-emulsification time is determined by using USP dissolution apparatus 2 at 50 rpm. 0.5 g of SEDDS formulations is introduced into 250 ml of 0.1N HCL or 0.5% Sodium Lauryl Sulphate(SLS) solution. The time for emulsification at room temperature is indicated as self-emulsification time for the formulation
10. In vitro Diffusion study:
This study is done to find release behavior of formulation using dialysis technique . Dialyzing medium generally used here is phosphate buffer (pH 6.8) .
One end of the dialysis membrane is tied with a thread and 1 ml of the SEDDS formulation along with 0.5 ml of dialyzing medium are filled in the membrane. The other end of membrane is also tied with thread and then allowed to rotate in the dialyzing medium at 100 rpm using dissolution apparatus or magnetic stirrer. Samples are withdrawn at regular time intervals.Samples are analyzed after suitable dilution . Volume of samples withdrawn is replaced with same amount of fresh dialyzing medium.
11. In vitro Dissolution technique:
The quantitative in vitro dissolution studies are carried out to assess drug release from oil phase into aqueous phase by USP type 2 dissolution apparatus using 500 ml of simulated gastric fluid containing 0.5% w/v of Sodium Lauryl Sulphate (SLS) at 50 rpm and the temperature is maintained at 37±0.5ºC. Aliquots of samples are withdrawn at regular intervals of time and volume withdrawn is replaced with fresh dissolution medium. Samples taken are then analyzed by UV spectrophotometer .
12. Liquefaction Time:
This particular test is performed to find the time required by solid SEDDS formulation to melt in vivo in simulated gastric fluid (in the absence of agitation). The formulation is packed in a polyethylene film(transparent) and tied to the bulb of thermometer. The thermometer is then placed in round bottom flask which is filled with simulated gastric fluid without pepsin. The temperature is maintained at 37±0.5ºC by using heating mantle.
13. Refractive index (R.I.) and Percent Transmittance:
Refractive Index and percent transmittance are used to check the transparency of formulation. Refractive Index(R.I) of the formulation is measured by refractometer by placing drop of solution on slide and is compared with water (R.I = 1.333). The percent transmittance of the formulation is measured at a particular wavelength using UV spectrophotometer .Distilled water is used as blank.
If R.I. of formulation is similar to that of water & formulation having percent transmittance is greater than 99%, then the formulation are transparent in nature. (27-32)
Different types of SEDDS formulations
1. Self-Emulsifying Capsules:
Capsule having conventional liquid self-emulsifying formulation on administration spontaneously form droplets of micro emulsion & then disperse in gastro intestinal tract (GIT) resulting in improved absorption. They have certain limitations as drug absorption decreases due to irreversible phase separation of microemulsion. In such case to increase the absorption, sodium dodecyl sulphate is added to self emulsifying formulations & super-saturable SEDDS is formulated by using a small quantity of polymer in the formulation to prevent drug precipitation by generating and maintaining supersaturated state in vivo. These formulations contain a reduced amount of surfactant & minimize any gastrointestinal side effects
2. Dry Emulsion:
o/w emulsion is converted into solid by spray drying, using solid carrier adsorption or freeze drying technique. Dry emulsion may be redispersed in water before use. In these powders emulsification occurs spontaneously in vivo or after exposure to aqueous solution. Dry emulsion technology effectively removes the stability problems (such like creaming ,phase separation & contamination by micro- organism during storage) associated with classic emulsion and avoids the use of harmful or toxic organic solvents .
MCT (Medium Chain Triglycerides) are generally used as oil phase for these formulations. Dry emulsions can be used for further preparation of tablets & capsules.
3. Self-Emulsifying Solid Dispersion:
Stability is a major concern during manufacturing of self emulsifying solid dispersion. Solid dispersions had widely being used to increase the rate of dissolution and bioavailability of poorly water soluble drugs. Hot-melt granulation is a widely used method for the preparation of solid dispersion.
4. Self-Emulsifying Tablets:
Preparation of Self Emulsifying Tablets involved adsorption of nanoemulsion on granular materials and then they are compressed to form tablets.
5. Self-Emulsifying Beads:
In Self emulsifying systems, solid dosage forms can be developed by formation of Beads i.e by using less amount of excipient. SE system is incorporated into micro porous polystyrene beads using solvent evoparation method by Paradkar & Patil . Porous polystyrene beads having complex internal void structures are produced by copolymerization of monomers styrene and divinyl benzene. It is chemically stable ,inert and biocompatible over a wide range of pH, temperature &humidity. The loading efficiency and in vitro drug release from self emulsifying system loaded porous poly styrene beads is governed by Geometrical features of porous materials like bead size & pore architecture .
6. Self-Emulsifying Nanoparticles:
It can be prepared by sonication ,solvent injection method,emulsion-diffusion-evaporation method. In solvent injection method molten lipid mass with lipid, surfactant & drug is injected drop by drop into a non-solvent system. Particles that are larger in size are removed by filtration and then the filtrate is dried to get nanoparticles. (33-34)
Solidification techniques for Transforming Liquid/Semisolid: Various solidification techniques are as follows
1.Capsule filling:
For oral route Capsule filling is simplest and the most common technology for the encapsulation of liquid or semisolid Self emulsifying formulations. For semisolid formulations, it is a four-step process (35) :
A) Heating excipient of semisolid to at least 20˚C above its melting point.
B) Incorporating the active substances with stirring.
C) Capsule filling with the melt cooling to room temperature. For liquid formulations, it involves a two-step process.
D) Filling of the formulation into the capsules followed by sealing of the body and cap of the capsule, either by micro spray sealing or by banding
2. Spray Drying:
This technique involves the preparation of a formulation by mixing lipids, surfactants, drug, solid carriers and solubilization of the mixture prior to spray drying. The solubilized liquid form is then atomized into a spray of droplets. The droplets are introduced into a drying chamber. volatile vehicles used evaporate leaving behind small solid particles which may be compressed into either tablets or filled into capsules (36).
2. Spray Cooling:
This technique is also known as spray congealing. In spray cooling molten formulation is prepared by mixing lipids, surfactants, and drug. Then mixture is sprayed into a cooling chamber. The molten droplets congeal and recrystallize into spherical solid particles which collect at the bottom of the chamber in the form of fine powder. The fine powder is used for development of solid dosage froms such as capsules, tablets etc. To atomize the liquid mixture and to generate droplets, different atomizers can be used but the most prefered one ultrasonic atomizer . The excipients used with this technique are polyoxyl glycerides specially steroylpolyoxyl glycerides, gelucire 50/13 (37)
3. Adsorption to Solid Carriers:
Adsorption to solid carriers is done by adding liquid SEDDS onto the solid carriers by mixing in a blender. Solid carriers can be micro porous inorganic substances with high surface-area colloidal inorganic adsorbent substances, cross-linked Polymers or Nanoparticle adsorbents, for example, silica, silicates, magnesium trisilicate, magnesium hydroxide, talcum, Crosspovidone and then the resulting free powder may then be filled directly into capsules or, alternatively, mixed with suitable excipients before compression into tablets. A significant benefit of the adsorption technique is good content uniformity. (38)
4.Melt granulation
Melt granulation is a process in which agglomeration of powder is obtained through the addition of a binder that melts or softens at relatively lower temperature
5.Melt extrusion/extrusion spheronization
Melt extrusion is a solvent‑free process that allows high drug loading of 60% as well as content uniformity.[39] Extrusion is a procedure of product of uniform shape and density by forcing it through a die under controlled temperature, product flow and pressure conditions.[40]
Specific applications of SEDDS:
1. Oral bioavailability enhancement poorly water soluble drugs: In case of poorly water soluble drugs dissolution rate dependent absorption is a main factor that limits the bioavailability of drug. The ability of SEDDS to release the drug in to GIT and disperses as micro emulsified form (globule size between 1- 100 nm). As the globular size is so small , increase in surface area enables more efficient drug transport through the intestinal aqueous boundary layer and through the absorptive brush border membrane leading to increased bioavailability(41).
2. In delivery of Peptides: SEDDS can deliver macromolecules like peptides, hormones, enzyme substrates and inhibitors and hece these are protected them from enzymatic hydrolysis. These systems are formed spontaneously without use of energy or heating and hence suitable for thermo labile drugs such as peptides (42) e.g. the intestinal hydrolysis of pro-drug by cholinesterase can be protected if Polysorbate 20 is emulsifier in formulation of micro emulsion.
3. Supersaturable SEDDS (S-SEDDS) & reduction of side effect of Surfactant:
To achieve faster absorption of poorly soluble drug high surfactant concentration is used which may cause irritation of gastro intestinal tract. S-SEDDS formulations have a less level of surfactant along with a polymeric precipitation inhibitor which stabilize the drug in a state of super saturation.
HPMC & other polymers of cellulose inhibit crystallization and maintain supersaturated state of drug for longer duration. S-SEDDS formulation provides a better safety profile when compared to conventional SEDDS formulation.
The mechanism of inhibited crystal growth and stabilization of super saturation by means of polymers needs further explanation (43).
e.g. in SEDDS formulation of salicylic acid and docetaxel (44) , HPMC is used as precipitation inhibitor (44)
A fivefold increase in bioavailability has been observed with PNU-91325 when HPMC is used in place of propylene glycol as a precipitation inhibitor (45).
CONCLUSION:
Self emulsifying drug delivery systems are actually mixtures of drug, lipid phase, emulsifier and/or co-solvent. SEDDS are a better approach for drugs with poor aqueous solubility and hence can be more useful for BCS Class II and IV .
When the dosage form reaches Gastro instestinal tract , the SEDDS system absorb water from its surrounding environment and spontaneously forms oil in water emulsion which disperse into fine droplets. The finer droplets provide greater surface area for the drug to dissolve or permeate in surrounding medium. SEDDS are prepared generally in liquid dosage forms but solid SEDDS (tablets, capsules, beads, microspheres etc.) are preferred due to ease in handling, transportation and better stability.
SEDDS avoids gastro intestinal irritation . Through SEDDS controlled and sustained release of drug release is achievable.
REFERENCE:
1. http://shodhganga.inflibnet.ac.in/bitstream/10603/6436/6/06_chapter%201.pdf
2. Chouksey r, et al: Preparation and evaluation of the self-emulsifying drug delivery system containing atorvastatin HMGCOA inhibiter. International Journal of Pharmacy and Pharmaceutical Sciences 2011; 3(3): 147-152.
3. Cho SH, Kang BK, Lee JS. Self-microemulsifying drug delivery system for oral bioavailability enhancement, pharmaceutical research. Int J Pharm 2004;274:65-73.
4. Gursoy RN, Benita S. Self-emulsifying drug delivery systems for improved oral delivery of lipophilic drugs. Biomed Pharmacother 2006;8:173-82.
5.Bhupendra G. Prajapati and Madhabhai M. Patel.Conventional and alternative pharmaceuticalmethods to improve oral bioavailability of lipophilicdrugs. Asian journal of pharmaceuticssvolume 2007;1 Suppl 1: 1-8.
6.Vergote GJ, Vervaet C., Van DI, Hoste S., De SmedtS., Demeester J., Jain RA, Ruddy S, Remon JP. Anoral controlled release matrix pellet formulationcontaining nanocrystallineketoprofen. Int J Pharm.2001; 219 Suppl 1-2: 81-87.
7. Kumar S, Malviya R, and Sharma P K: Solid Dispersion: Pharmaceutical Technology for the Improvement of Various Physical Characteristics of Active Pharmaceutical Ingredient; African Journal of Basic and Applied Science 2011; 3(4): 116-125.
8. Kumar S, Gupta S and Sharma P K: Self-Emulsifying Drug Delivery Systems (SEDDS) for oral delivery of lipid based formulations. African Journal of Basic & Applied Science 2012; 4 (1): 07-11.
9. Nigade P M, Patil S, Tiwari S S: Self Emulsifying drug delivery system (SEDDS): A review. International Journal of Pharmacy and Biological Sciences 2012; 2(2): 42-52.
10. Meinzer A, Mueller E, Vonderscher J. Microemulsion–a suitable galenical approach for the absorption enhancement of low soluble compounds. B T Gattefosse 1995;88:21‑6.
11. Craig DQ, Barkar SA, Banning D, Booth SW. An investigation into the mechanisms of self‑emulsification using particle size analysis and low frequency dielectric spectroscopy. Int J Pharm 1995;144:103‑10.
12. Tenjarla S. Microemulsions: an overview and pharmaceutical applications. Crit Rev Therapeut Drug Carrier Syst 1999; 16: 461- 521.
13. Ghosh PK, Murthy RSR. Microemulsions: A potential drug delivery system. Curr Drug Deliv 2006; 3: 167-180.
14. Attwood, D, Mallon C, Taylor, CJ. 1992. Phase studies of oil-in water phospholipid microemulsions, Int. J. Pharm. 84, R5–R8.
15. Aboofazeli R, Lawrence CB, Wicks SR, Lawrence MJ. Investigations into the formation and characterisation of phospholipid microemulsions. III. Pseudo-ternary phase diagrams of systems containing water–lecithin–isopropyl myristate and either an alkanoic acid, amine, alkanediol, polyethylene glycol alkyl ether or alcohol as cosurfactant, Int. J. Pharm. 1994; 111, 63–72.
16. Lawrence MJ, Rees GD. Microemulsion based media as novel drug delivery systems. Adv Drug Del Rev 2000; 47: 89-121.
17. Stilbs P, Lindman B, Rapacki K. Effect of alcohol cosurfactant length on microemulsion structure. J Colloid Interface Sci 1983; 95: 583-585.
18. Shukla J B, et al: Self micro emulsifying drug delivery system pharma science monitor. Journal of Pharmacy and Pharmaceutical Sciences 2010; 1(2): 13-33.
19. BhargavaP, Bhargava S, and Daharwal S J: Self-emulsifying drug delivery System: an approach to improve the solubility of poorly water soluble drugs. Advance Research in Pharmaceuticals and Biologicals 2011; Vol 1(1): 1-9.
20. Christopher Porter J H, et al: Enhancing intestinal drug solubilization using lipid-based delivery systems. Advanced Drug Delivery Reviews 2008; 60: 673–691.
21. Kumar A, Sharma S, Kamble R: Self-emulsifying drug delivery system (SEDDS): future aspects. International Journal of Pharmacy and Pharmaceutical Sciences 2010; 2(4): 7-13.
22. Mittal P, Seth N, Rana AC: Self-microemulsifying drug delivery system (SMEDDS): An alternative approach for hydrophobic drugs. International Journal of Natural Product Science 2012; 1: 80.
23. Rang MJ, Miller CA. Spontaneous emulsification of oils containing hydrocarbon, nonionic surfactant and oleyl alcohol. J Colloid Interface
Sci 1999;209:179‑92.
24. Patel P A, et al: Self Emulsifying Drug Delivery System: A Review. Research Journal of Pharmacy and Technology 2008; 1(4): 313-323.
25. Revathi S, Dhana Raju MD: Self-emulsifying drug delivery system: A review. World Journal of Pharmacy and Pharmaceutical Sciences 2013; 2(1): 89-107.
26. Sunitha R, Satya sireesha D and Aparna M V: Novel self-emulsifying drug delivery system- an approach to enhance bioavailability of poorly water soluble drugs. International Journal of Research in Pharmacy and Chemistry 2011; 1(4): 828-838.
27. Singh G, et al: Self-emulsifying drug delivery systems (SEEDS): An approach for delivery of poorly water soluble drug. International Journal of Pharmacy & Life Sciences 2012; 3(9): 1991-1996.
28. Rajinikanth P S, Suyu Y, and Garg S: Development and In-Vitro Characterization of Self-nanoemulsifying Drug Delivery Systems of Valsartan. World Academy of Science, Engineering and Technology 2012; 72: 1418-1423.
29. Sachan R, Khatri K, Kasture S B: Self-Eumlsifying Drug Delivery System A Novel Approach for enhancement of Bioavailability. International Journal of PharmTech Research 2010; 2(3): 1738-1745.
30. Patil P, Patil V, Paradkar A: Formulation of SEDDS for oral delivery of Simvastatin: In vitro and in vivo evaluation. Acta pharma. 2007; 57: 111-122.
31. Kohli K, et al: Self-emulsifying drug delivery systems: an approach to enhance oral Bioavailability. Drug Discovery Today 2010; 15: 958-965.
32. Jang D J, et al: Improvement of bioavailability & photo stability of amlodipine using redispersible dry emulsion. Europian Journal of Pharmaceutical Science 2006; 28: 405-411.
33. Kawakami K: Modification of physicochemical characteristics of active pharmaceutical ingredients and application of supersaturatable dosage forms for improving bioavailability of poorly absorbed drugs. Advanced Drug Delivery Reviews 2012; 64: 480–495.
34. Patil P, Paradkar A: Porous polystyrene beads as carriers for Self emulsifying system containing Loratadine. American Association of Pharmaceutical Scientist Pharm Sci Tech 2006; 7(1): E1-E7.
35. Rajeshwar V and Shrivastava B: Self Emulsifying Drug Delivery System (SEDDS): A conventional and alternative approach to improve oral bioavilability. Int J Pharm Sci Res 2018; 9(8): 3114-27. doi: 10.13040/IJPSR.0975-8232.9(8).3114-27.
36.Yi T, et al: A new solid self-micro emulsifying formulation prepared by Spray drying to improve the oral bioavailability of poorly water soluble drugs. European Journal of Pharma and Biopharma 2008; 70: 439-444.
37. Cavallari C,et al: Thermal and Fractal analysis of diclofenac/Gelucire 50/13 micro particles obtained by ultrasound-assisted atomization. Journal of Pharmaceutical Sciences 2005; 94:1124-1134.
38. Khedekar and Mittal, SELF EMULSIFYING DRUG DELIVERY SYSTEM: A REVIEW .IJPSR, 2013; Vol. 4(12): 4494-4507.
39. Hauss DJ, Fogal SE, Ficorilli JV, Price CA, Roy T, Jayaraj AA, et al. Lipid‑based delivery systems for improving the bioavailability and lymphatic transport of poorly water‑soluble LTB4 inhibitor. J PharmSci 1998;87:164‑9.
40. Pillay V, Fassihi R. Unconventional dissolution methodologies. J Pharm Sci 1999;88:843‑51.
42. Xiaole Qi, et al: Self-double-emulsifying drug delivery system (SDEDDS): A new way for oral delivery of drugs with high solubility and low permeability. International Journal of Pharmaceutics 2011; 409(1-2): 245–251. \
43. Kyatanwar AU, et al: Self micro-emulsifying drug delivery system (SMEDDS): Review. Journal of Pharmacy Research 2010; 3(1):75-83.
44. Chen Y, et al: Development of a solid S-SEDDS of docetaxel with improved dissolution & bioavailability. Biological and Pharmaceutical Bulletin 2011; 34: 278-286.
45. Mishra N, Srivastava S: New Strategy for Solubilization of poorly soluble drug- SEDDS. Der Pharmacia Lettre 2009; 1 (2): 60-67.